GE Healthcare 6960-MON Novii Intrapartum Maternal/Fetal monitor Pod User Manual 107 TF 100 USrev7 Novii Technical Datasheet
GE Healthcare Novii Intrapartum Maternal/Fetal monitor Pod 107 TF 100 USrev7 Novii Technical Datasheet
Contents
107-TF-100-USrev7_Novii Technical Datasheet

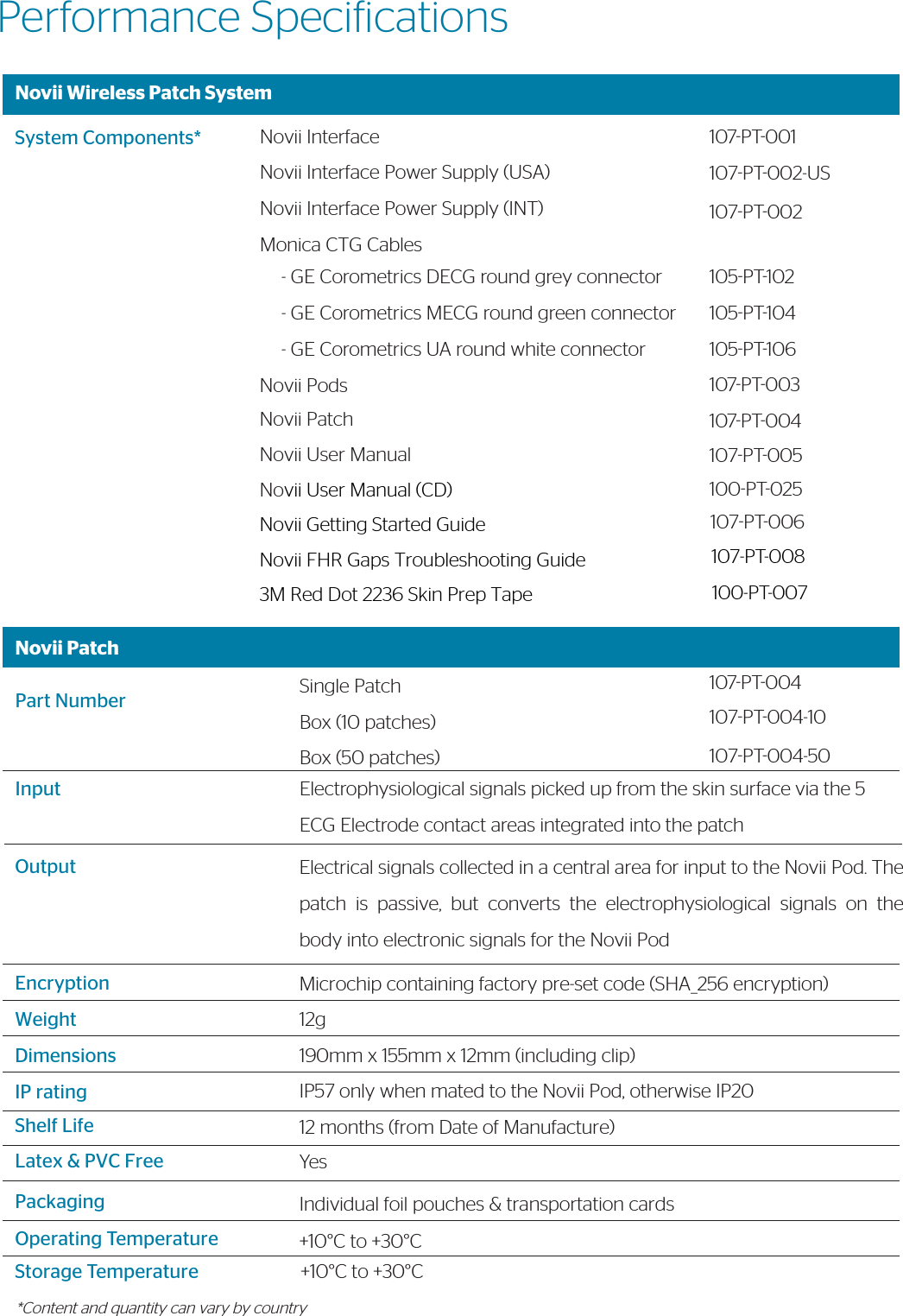
![Novii PodPart NumberOperating ModeBluetooth WirelessUser InterfaceFHRMHRUAPower WeightDimensionsIP ratingAccessoriesOperating TemperatureStorage TemperatureType107PT003Real-Time/ Continuous UseOutput ProtocolRange LEDRange:Resolution:Accuracy:Range:Resolution:Accuracy:Range:Resolution:Accuracy:BatteryBattery LifeBattery Charging40g45mm x 39mm x 20mm (including contact pins)IP57 only when mated to the Novii Patch, otherwise IP20Novii Patch (107PT004)+10°C to +30°C+10°C to +30°CType BF Equipment (applied part is the Novii patch, which connects to the pod via the spring contact pins at the bottom of the pod)Bluetooth v2.1 + EDR, Class 1.5, to Novii InterfaceModiied Series 50 30m (line of sight) 60 -240 BPM ¼ BPM, 4 times/ second, rolling 2 sec averageBland Altman vs AN24 predicate 7.08BPM rms [1] 40 -240 BPM ¼ BPM, 4 times/ second, rolling 2 sec averageBland Altman vs AN24 predicate 5.32BPM rms [2]0 – 500 microvolts0 – 255 levels representing 100% of full scale, 4 times/ second, rolling 2 second average97.99% percent agreement (interpretability) 86.05% Positive Percent Agreement (Sensitivity)Rechargeable Lithium Polymer 3.7V, 750mAh80% capacity after 475 charge cyclesUp to 11 hrsContactless via the Novii Interface. Charge time for x2 fully discharged Pods – up to 2 hours](https://usermanual.wiki/GE-Healthcare/6960-MON.107-TF-100-USrev7-Novii-Technical-Datasheet/User-Guide-3677764-Page-3.png)